Page 77 - 048
P. 77
59
operating time. Resulting in progressively decreased in acetate produced and/or accumulated in
the reactor and pH in CH 4-UASB reactor ranged 7.43-7.90 as shown in Fig. 4.9. Best known
some species of methanogens such as Methanosaeta has lower growth rate due to affected by
acetate concentration. Moreover, a study from Kongjan et al. (2014) found that acetic acid was
the major soluble metabolite products and it’s produced in relatively high concentration of 51±5
mM achieved from sole fermentation of SLS in UASB reactor. These corresponded to a study
from Boe (2006) reported that acetate oxidation pathway become more favorable under
thermophilic temperatures. Therefore, one explanation for low methane production yield is that
methanogens was inhibited by a very high concentration of acetate produced and accumulated in
the reactor. Moreover, other possible reasons are: (1) low methane producing archaea in the CH 4-
UASB reactor; (2) inhibitants in SLS and POME such as sulfate and phenolic compounds; (3)
high hydrogen partial pressure; (4) the competition of sulfate reducing bacteria with
methanogenic archaea which could occur under low concentrations of sulfate (Boe, 2006).
Reduced product especially lactate was broken dawn to acetate, carbon dioxide and bicarbonate
which was performed by two groups of sulfate reducing bacteria including incomplete and
complete oxidizers (Chen et al., 2008).
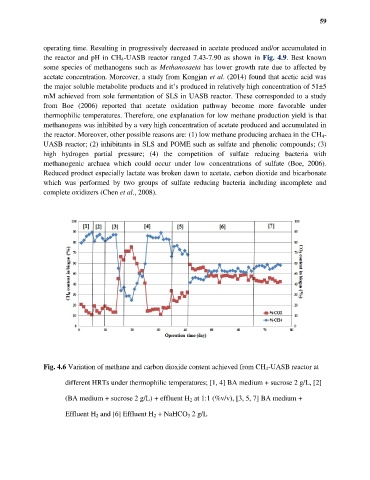
Fig. 4.6 Variation of methane and carbon dioxide content achieved from CH 4-UASB reactor at
different HRTs under thermophilic temperatures; [1, 4] BA medium + sucrose 2 g/L, [2]
(BA medium + sucrose 2 g/L) + effluent H 2 at 1:1 (%v/v), [3, 5, 7] BA medium +
Effluent H 2 and [6] Effluent H 2 + NaHCO 3 2 g/L