Page 54 - 048
P. 54
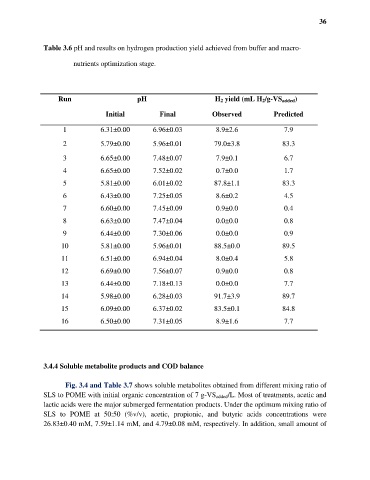
36
Table 3.6 pH and results on hydrogen production yield achieved from buffer and macro-
nutrients optimization stage.
Run pH H 2 yield (mL H 2/g-VS added)
Initial Final Observed Predicted
1 6.31±0.00 6.96±0.03 8.9±2.6 7.9
2 5.79±0.00 5.96±0.01 79.0±3.8 83.3
3 6.65±0.00 7.48±0.07 7.9±0.1 6.7
4 6.65±0.00 7.52±0.02 0.7±0.0 1.7
5 5.81±0.00 6.01±0.02 87.8±1.1 83.3
6 6.43±0.00 7.25±0.05 8.6±0.2 4.5
7 6.60±0.00 7.45±0.09 0.9±0.0 0.4
8 6.63±0.00 7.47±0.04 0.0±0.0 0.8
9 6.44±0.00 7.30±0.06 0.0±0.0 0.9
10 5.81±0.00 5.96±0.01 88.5±0.0 89.5
11 6.51±0.00 6.94±0.04 8.0±0.4 5.8
12 6.69±0.00 7.56±0.07 0.9±0.0 0.8
13 6.44±0.00 7.18±0.13 0.0±0.0 7.7
14 5.98±0.00 6.28±0.03 91.7±3.9 89.7
15 6.09±0.00 6.37±0.02 83.5±0.1 84.8
16 6.50±0.00 7.31±0.05 8.9±1.6 7.7
3.4.4 Soluble metabolite products and COD balance
Fig. 3.4 and Table 3.7 shows soluble metabolites obtained from different mixing ratio of
SLS to POME with initial organic concentration of 7 g-VS added/L. Most of treatments, acetic and
lactic acids were the major submerged fermentation products. Under the optimum mixing ratio of
SLS to POME at 50:50 (%v/v), acetic, propionic, and butyric acids concentrations were
26.83±0.40 mM, 7.59±1.14 mM, and 4.79±0.08 mM, respectively. In addition, small amount of